The Importance of Chelating Agents
One aspect of formulating that many people overlook is the importance of neutralizing minerals in your product. Sometimes it can’t be helped. You may have clay as an ingredient, or you may have intentionally added something like magnesium sulfate or sea salt for a texturizing spray. Those niche cases aside, a chelating agent should be something that every water-product design attempts to incorporate.
Why Your Formula Needs A Chelating Agent

Minerals (dissolved metals and metal ions) are usually not great news for product shelf life. They can cause colors to fade. Also, many microbes love iron, just like we do; they need it to grow. If you have large amounts of iron mineral floating in solution then the microbes have ready access to an essential nutrient. Additionally, minerals like calcium and magnesium can react with soaps to form “soap scum” – a hard-to-dissolve deposit that reduces the effectiveness of your product and can be unsightly. While using deionized water in formulations is certainly a good “first step”, minerals can find their way into the product in a number of ways. They might leach from the container, depending on the container’s choice. They might “come along for the ride” with botanical extracts like aloe vera, a natural source of minerals. The customer might accidentally contaminate the bulk of the product.
How Chelating Agents Work
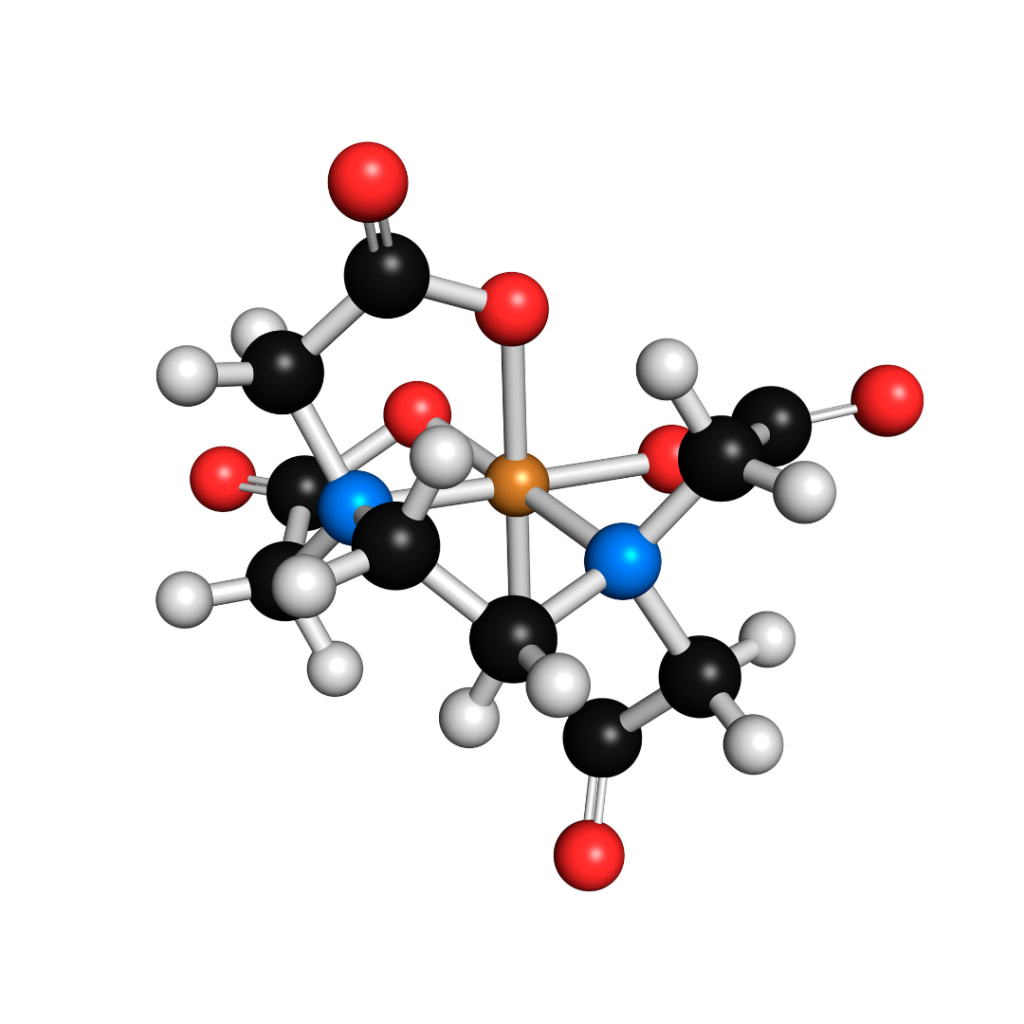
Successful product preservation is vital for preventing possible litigation against your formulating company. For that reason, I always advise adding a chelating agent to water-containing formulas. “Chelate” comes from the Latin word for “claw”, and it’s pretty appropriate. Chelating agents have multiple “arms”, almost like claws, extending from the center of the molecule. Each of the tips of the arms has a high affinity for minerals. So, by adding a chelating agent you’re releasing mineral-neutralizing molecules into your formula. If a chelating agent bumps into a mineral ion, it will “wrap around”, or chelate, the mineral ion. It’s almost like gripping a baseball in your hand. It also sticks pretty well, so that mineral ion can no longer cause problems in your formula.
EDTA – The Gold Standard
There are a number of chelating agents that all work fairly well. The gold standard in cosmetics is EDTA. It was custom-built by chemists to have just the right size cavity for the mineral ion, and just the right binding strength, and therefore it’s one of the most effective chelators. Usually, around 0.2% of EDTA (in its various salt forms), dissolved into water at the start of the formulation, is sufficient. However, EDTA has a glaring drawback: it doesn’t biodegrade. It inevitably finds its way into the water system, where it can help mobilize deposits of less-healthy metals downstream. This can cause environmental difficulties.
EDTA Alternatives
Despite EDTAs excellent performance as a chelator, this lack of biodegradability has formulators looking for alternative chelating agents. I have three to suggest, all of which biodegrade and yet also perform the chelating duties while in the product bottle.
1. Sodium Phytate
The first is sodium phytate. This is present in high amounts in rice bran. It’s a white powder that has excellent water solubility and good biodegradability. I normally recommend the same amount as EDTA – 0.2%. Used in higher amounts it begins to have other beneficial properties, but in the interest of “less is more”, I tend to use just the amount needed for successful chelation. Because it’s very naturally sourced, customers looking for a plant-based solution will be pleased with the choice of this chelator.
2. Sodium Gluconate.
The second is sodium gluconate. This is an oxidized form of glucose (gluconic acid) that has been neutralized so that it’s not acidic. Another white powder, easily dissolves in the water phase. It is also biodegradable. Sodium gluconate does not have the optimal size and shape to be as effective as EDTA. However, raising the loading level to 0.5% can help to compensate for this.
3. Tetrasodium Glutamate Diacetate
The third option is tetrasodium glutamate diacetate. Its molecule looks very similar to EDTA, and at a loading level of 0.5-1%, it can approach EDTAs performance. However, unlike EDTA it biodegrades. It would be a good option when you need EDTA-level chelation but want to use eco-responsible ingredients.
Improved colour and fragrance stability
Chelators have other beneficial properties besides just tying up random mineral ions. They can help to prevent fragrance fading and color loss. Used in higher amounts in clarifying shampoos, they can help strip unwanted materials from the scalp/hair that might have built up after swimming (whether chloride from a pool or minerals from the ocean).
Conclusion
Sometimes when formulating products, it’s easy to get caught up in the excitement of the various actives that you are using and to lose focus of the product’s skeleton. I always recommend getting the structural framework of a product in place first (including antioxidant, chelating agent, and broad-spectrum preservative). Maintaining that focus will help your product stay fresh for longer.

